Get Drug Screen Form in PDF
The Drug Screen form is a crucial document in the process of drug testing, particularly in workplace settings. It serves as a comprehensive record that details the collection and testing of a specimen. This form includes essential information such as the employer's name and address, the medical review officer's contact details, and the donor's identification number. Additionally, it outlines the testing authority, which can range from the Department of Transportation to Health and Human Services, ensuring that the appropriate regulations are followed. The reasons for testing are clearly specified, whether it be for pre-employment, random checks, or post-accident evaluations. Furthermore, the form lists the specific drug tests to be performed, including substances like THC, cocaine, and opiates. Each step of the collection process is meticulously documented, from the collector's observations regarding specimen temperature to the chain of custody procedures that guarantee the integrity of the sample. The form also includes sections for reporting results, whether negative or positive, and addresses any issues such as adulteration or invalid results. By providing a structured approach to drug testing, the Drug Screen form plays a vital role in maintaining safety and compliance in various environments.
Dos and Don'ts
Filling out a Drug Screen form is a critical process that requires attention to detail. Here are some essential dos and don’ts to keep in mind.
- Do ensure that all personal information is accurate and complete.
- Do specify the testing authority clearly, selecting from the provided options.
- Do indicate the reason for the test, as this helps in categorizing the results.
- Do check the temperature of the specimen within the specified time frame.
- Don't leave any fields blank; incomplete forms can lead to delays.
- Don't provide incorrect or misleading information; honesty is crucial.
- Don't forget to sign and date the form where required; your signature is a legal affirmation.
- Don't neglect to keep a copy of the completed form for your records.
By following these guidelines, you can help ensure a smooth testing process and avoid complications that may arise from errors or omissions.
Document Attributes
| Fact Name | Description |
|---|---|
| Form Purpose | The Drug Screen form is used for documenting the collection and testing of urine specimens for drugs. |
| Federal Oversight | This form is governed by federal regulations, specifically the Department of Transportation (DOT) and the Department of Health and Human Services (HHS). |
| Specimen Identification | Each specimen is assigned a unique ID number to ensure proper tracking and handling throughout the testing process. |
| Testing Authorities | The form allows for specification of testing authorities, including DOT agencies such as FMCSA and FAA. |
| Reasons for Testing | Common reasons for testing include pre-employment, random checks, reasonable suspicion, and post-accident scenarios. |
| Drug Panel | Tests can detect various substances, including THC, cocaine, PCP, and amphetamines, among others. |
| Chain of Custody | The form includes a chain of custody section to document the handling of the specimen from collection to testing. |
| Collector's Responsibilities | The collector must ensure the specimen's temperature is recorded and that it falls within the acceptable range. |
| Results Reporting | Test results are reported as negative, positive, or rejected, with specific substances identified when positive. |
| Compliance | All procedures must comply with applicable federal requirements to ensure the integrity of the testing process. |
Key takeaways
When filling out and using the Drug Screen form, it is essential to follow specific guidelines to ensure accuracy and compliance. Here are some key takeaways:
- Accurate Information: Ensure that all fields are completed with precise details, including the employer's name, address, and identification number.
- Testing Authority: Clearly specify the testing authority, whether it is HHS, NRC, or DOT, and include the relevant DOT agency if applicable.
- Reason for Testing: Indicate the reason for the test accurately. This could be for pre-employment, random selection, reasonable suspicion, post-accident, return to duty, or follow-up.
- Drug Tests Selection: Select the appropriate drug tests to be performed. Options include THC, COC, PCP, OPI, and AMP, among others.
- Temperature Check: The collector must read the specimen temperature within four minutes of collection. Ensure that it falls between 90° and 100° F.
- Chain of Custody: Maintain a clear chain of custody by ensuring that the specimen is collected, labeled, sealed, and released according to federal requirements.
- Signatures Required: Both the collector and the certifying scientist must sign the form to validate the process. This includes documenting the date and time of collection.
Following these guidelines will help ensure that the Drug Screen form is filled out correctly and that the testing process is conducted in compliance with applicable regulations.
Other PDF Templates
Medicare Deduction From Social Security - Completing the SSA-44 can significantly decrease your monthly Medicare premium expenses.
A Texas Hold Harmless Agreement is a legal document designed to protect one party from liability for any injuries or damages that may occur during a specific activity or event. This form outlines the responsibilities of each party involved and ensures that the party being held harmless will not be held accountable for certain claims. Individuals and organizations looking to create this important document can find resources at Texas Forms Online, which can help them mitigate risks effectively.
Roofing Inspection Template - Describe the building type to understand the roof's design requirements.
Example - Drug Screen Form
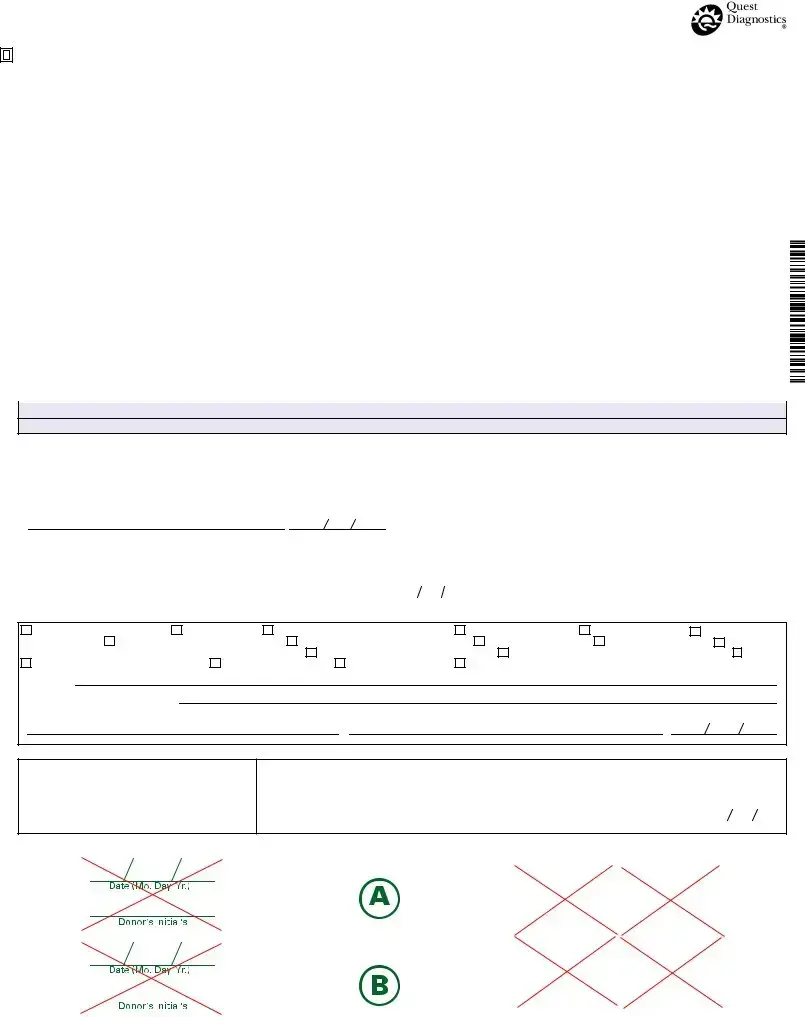
FEDERAL DRUG TESTING CUSTODY AND CONTROL FORM
SPECIMEN ID NO. |
|
STEP 1: COMPLETED BY COLLECTOR OR EMPLOYER REPRESENTATIVE |
LAB ACCESSION NO. |
Quest, Quest Diagnostics, the associated logo and all associated Quest Diagnostics marks are the trademarks of Quest Diagnostics Incorporated. © Quest Diagnostics Incorporated. All rights reserved.
A. Employer Name, Address, I.D. No. |
|
|
B. MRO Name, Address, Phone and Fax No. |
||||||||||
|
|
|
|
|
|
|
|
|
|||||
C. Donor SSN or Employee I.D. No. _______________________________________________________________ |
|
|
|
|
|||||||||
D. SpecifyTesting Authority: HHS |
NRC |
DOT – Specify DOT Agency: FMCSA |
FAA |
FRA FTA PHMSA USCG |
|||||||||
E. Reason forTest: |
Random |
Reasonable Suspicion Cause Post Accident |
Return to Duty |
|
|||||||||
F. DrugTests to be Performed: |
THC, COC, PCP, OPI, AMP |
THC & COC Only |
Other (specify) ________________________________________________ |
||||||||||
G. Collection Site Name: |
|
|
|
|
|
Collection Site Code: |
|
|
|
|
|||
Address: |
|
|
|
|
|
|
Collector Phone No.: |
|
|
||||
City, State and Zip: |
|
|
|
|
|
Collector Fax No.: |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
STEP 2: COMPLETED BY COLLECTOR (make remarks when appropriate) Collector reads specimen temperature within 4 minutes.
Temperature between 90° and 100° F? Yes No, Enter Remark |
Collection: Split Single None Provided, Enter Remark |
Observed, (Enter Remark) |
REMARKS
STEP 3: Collector affixes bottle seal(s) to bottle(s). Collector dates seal(s). Donor initials seal(s). Donor completes STEP 5 on Copy 2 (MRO Copy)
STEP 4: CHAIN OF CUSTODY - INITIATED BY COLLECTOR AND COMPLETED BY TEST FACILITY
|
I certify that the specimen given to me by the donor identified in the certification section on Copy 2 of this form was |
|
SPECIMEN BOTTLE(S) RELEASED TO: |
|||||||
|
collected, labeled, sealed, and released to the Delivery Service noted in accordance with applicable Federal requirements. |
Quest Diagnostics Courier |
||||||||
|
|
X |
|
|
|
|
|
FedEx |
||
|
|
Signature of Collector |
|
|
|
|
|
Other |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AM |
|
|
|
|
|
|
|
|
|
|
PM |
|
|
|
|
|
|
(Print) Collector's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
|
Time of Collection |
|
|
Name of Delivery Service |
||
RECEIVED AT LAB OR IITF: |
|
|
|
|
|
Primary Specimen |
SPECIMEN BOTTLE(S) RELEASED TO: |
|||
|
X |
|
|
|
|
|
Bottle Seal Intact |
|
||
|
|
|
|
|
|
Yes No |
|
|||
|
|
Signature of Accessioner |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
If No, Enter remarks |
|
|
|
|
|
|
|
|
|
|
in Step 5A. |
|
|
|
|
(Print) Accessioner’s Name (First, MI, Last) |
|
|
|
Date (Mo./Day/Yr.) |
|
|||
STEP 5A: PRIMARY SPECIMEN REPORT - COMPLETED BY TEST FACILITY
NEGATIVE |
POSITIVE for: |
Marijuana Metabolite ( |
6- Acetylmorphine |
Methamphetamine |
MDMA |
|
DILUTE |
|
|
Cocaine Metabolite (BZE) |
Morphine |
Amphetamine |
MDA |
|
|
|
PCP |
Codeine |
|
MDEA |
REJECTED FOR TESTING |
ADULTERATED |
SUBSTITUTED |
INVALID RESULT |
|
|
|
REMARKS:
Test Facility (if different from above):
I certify that the specimen identified on this form was examined upon receipt, handled using chain of custody procedures, analyzed, and reported in accordance with applicable Federal requirements.
X
Signature of Certifying Scientist |
(Print) Certifying Scientist's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
STEP 5b: COMPLETED BY SPLIT TESTING LABORATORY
RECONFIRMED FAILED TO RECONFIRM - REASON ____________________________________________
___________________________________________ |
I certify that the split specimen identified on this form was examined upon receipt, handled using chain of custody |
||||||||||||||||||||||||||||
procedures, analyzed and reported in accordance with applicable Federal requirements. |
|
|
|
|
|
||||||||||||||||||||||||
|
|
|
|
|
|
|
Laboratory Name |
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
___________________________________________ |
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
Signature of Certifying Scientist |
|
|
|
(Print) Certifying Scientist's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
|||||||||||||||||||||||
|
|
|
|
|
|
|
Laboratory Address |
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OMB No.
PRESS HARD - YOU ARE MAKING MULTIPLE COPIES
Detailed Instructions for Writing Drug Screen
After gathering the necessary information, you can proceed to fill out the Drug Screen form. This form requires specific details about the donor and the testing process. Ensure that all sections are completed accurately to avoid any delays in processing.
- Start with the section labeled "COMPLETED BY COLLECTOR OR EMPLOYER REPRESENTATIVE." Fill in the Employer Name, Address, and I.D. No..
- Provide the MRO Name, Address, Phone, and Fax No..
- Enter the Donor SSN or Employee I.D. No..
- Specify the Testing Authority by checking the appropriate box (HHS, NRC, DOT) and, if applicable, specify the DOT Agency.
- Indicate the Reason for Test by checking one of the options provided or writing in another reason.
- List the Drug Tests to be Performed by checking the appropriate boxes or specifying other tests.
- Fill in the Collection Site Name, Collection Site Code, Address, Collector Phone No., City, State and Zip, and Collector Fax No..
- In the section for the collector, check if the specimen temperature is between 90° and 100° F and record any remarks.
- Indicate whether the collection was Split, Single, or None Provided and add any remarks as necessary.
- Affix the bottle seal(s) to the specimen bottle(s), date the seal(s), and have the donor initial the seal(s).
- Ensure the donor completes STEP 5 on Copy 2 (MRO Copy).
- Complete the CHAIN OF CUSTODY section by certifying that the specimen was collected and handled according to Federal requirements.
- Sign and print the Collector's Name and enter the Date and Time of Collection.
- Record the name of the Delivery Service used for transporting the specimen.
- In STEP 5A, the test facility will report the results, indicating whether the result is NEGATIVE or POSITIVE for specified substances.
- If applicable, fill out STEP 5B regarding split testing laboratory results and reasons for any failures to reconfirm.
- Finally, ensure all signatures are completed, including the Certifying Scientist's name and date.
Documents used along the form
When conducting drug screenings, several forms and documents are often utilized alongside the Drug Screen form to ensure compliance, maintain proper records, and facilitate communication among all parties involved. Below is a list of common forms that accompany the Drug Screen form, each serving a specific purpose in the drug testing process.
- Chain of Custody Form: This document tracks the handling of the specimen from the moment it is collected until it is tested. It ensures that the sample has not been tampered with and provides a record of who handled the specimen at each stage.
- Consent Form: Before testing, donors must provide written consent for the collection and testing of their samples. This form informs the donor of their rights and the procedures involved in the testing process.
- Medical Review Officer (MRO) Report: After the lab tests the specimen, the MRO evaluates the results, especially in cases of positive findings. This report includes the MRO’s interpretation and recommendations regarding the results.
- Employer Notification Form: This form is used by the testing facility to notify the employer about the test results. It typically includes details about the outcome and any follow-up actions that may be required.
- California ATV Bill of Sale Form: For a proper transfer of ownership, utilize the essential ATV Bill of Sale form required in California to ensure a legally recognized transaction.
- Test Facility Report: This document provides a detailed account of the testing performed, including the methods used and the final results. It is essential for maintaining accurate records and ensuring compliance with regulatory standards.
- Donor Identification Form: This form verifies the identity of the donor at the time of specimen collection. It helps prevent mix-ups and ensures that the correct individual is being tested.
- Split Sample Testing Form: In cases where a split sample is collected, this form is used to document the procedures followed for testing the second portion of the specimen, especially if the initial test results are disputed.
- Incident Report Form: If there are any unusual occurrences during the testing process, such as a donor refusing to provide a sample, this form documents the incident for future reference and compliance audits.
- Follow-Up Testing Authorization Form: If an employee tests positive, this form is used to authorize any subsequent tests required as part of the follow-up process, ensuring that all parties are informed and in agreement.
Utilizing these forms in conjunction with the Drug Screen form helps create a comprehensive framework for drug testing. This framework not only safeguards the rights of the individuals being tested but also upholds the integrity of the testing process, ensuring that it is conducted fairly and transparently.
